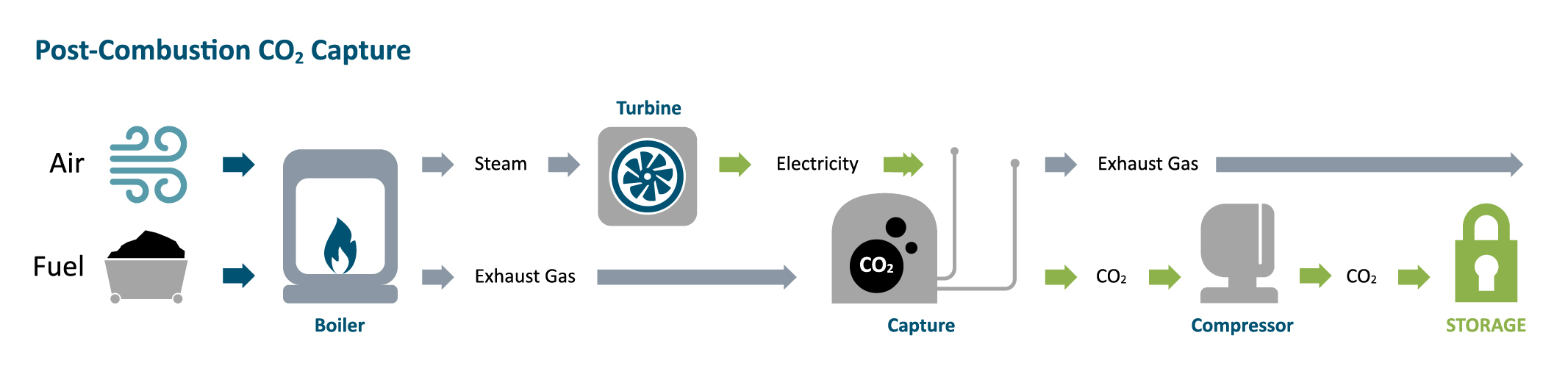
Carbon capture and storage (CCS) processes capture carbon dioxide (CO₂) emissions from industrial processes, power generation, and other sources, and then store them underground. Although it offers significant potential to reduce greenhouse gas emissions, as a relatively new technology, CCS faces several challenges.
Key carbon capture and storage challenges include cost, technical difficulties, safety, storage capacity, and regulatory requirements. Public perception can also be an issue, with critics raising concerns about its safety and effectiveness. In addition, CCS is sometimes seen as detracting from efforts to reduce emissions through renewable energy and better energy efficiency.
Commercial challenges
As a relatively new and expensive technology, CCS faces several commercial challenges that have limited its widespread adoption. The current cost of capturing and storing CO₂ can be prohibitively high, particularly for smaller industrial facilities or power plants. As a result, many companies are, at present, hesitant to invest in CCS technology due to concerns about its economic viability. While some governments have provided funding for CCS projects, many companies argue that additional incentives, such as tax credits or subsidies, are needed.
Storage challenges
Captured CO₂ is typically stored underground, usually in former oil and gas reservoirs which have been proven to have held their resources in place for millions of years. The capacity of these underground geological formations to store CO₂ is limited, and not all sites may be suitable for long-term storage. There is also a need to identify and assess potential storage sites, which can be time-consuming and expensive.
Infrastructure challenges
Suitable storage sites can be remote, so captured CO₂ may need to be transported across large distances, requiring a network of pipelines. The cost of building and maintaining these pipelines can be high, and there may be public resistance to their construction. These cost issues may also apply to the storage infrastructure itself, as even existing geological sites are likely to need to be adapted for the safe storage of CO₂ and monitored to ensure there is no leakage. Additionally, CCS infrastructure must be integrated with existing infrastructure, such as power plants or industrial facilities, which can be a complex and expensive undertaking.
CO₂ and its impact on material
Carbon dioxide can have significant adverse effects on materials, including corrosion, degradation, scaling, and embrittlement. These effects can be seen in a variety of industries, including oil and gas, power generation, and transportation, and so are likely to create challenges for any CCS process. Understanding and mitigating these effects is essential for maintaining safe and efficient operations.
Corrosion
When CO₂ reacts with water, it can form carbonic acid. So, when moisture contaminates pipelines transporting CO₂, it can lead to corrosion in metal components and pipes, particularly those made from carbon steel or copper alloys. This, in turn, can lead to leakage, which is often difficult to detect, and can be dangerous and expensive to repair.
Degradation
The presence of CO₂ can cause the degradation of materials such as polymers and rubber. Again, this is often caused by CO₂ reacting with moisture to create carbonic acid, and leads to an adverse effect on the material’s mechanical strength, stiffness, and other properties. CO₂ can also react with the calcium hydroxide present in concrete, leading to degradation and cracking.
Scaling
Scaling is the build-up of mineral deposits on equipment surfaces. When CO₂ dissolves in water, it can react with minerals to form scale. This can occur in pipelines, heat exchangers, and other equipment, leading to reduced efficiency and increased maintenance costs. Calcium-based materials, such as limestone and concrete used in storage sites, are also susceptible to scaling in the presence of CO₂.
Embrittlement
When CO₂ dissolves in some materials, particularly polymers and elastomers, it can cause them to become brittle and more prone to cracking or breaking under stress. This can affect components such as seals, gaskets, and coatings.